Acute Lymphoblastic Leukemia (ALL) is the most common cancer in children. ALL is an aggressive hematological cancer arising from the precursors of B and T lymphocytes, i.e. cells that should develop into normal lymphocytes and participate in the immune response. ALL arises due to abnormalities in these cells, which disturb the process of their maturation and excessively stimulate their proliferation. Lymphocyte precursors are ‘arrested’ in their maturation (they fail to perform a function in the immune response), and at the same time they proliferate in an uncontrolled manner.
ALL can develop from B-cell precursors (BCP-ALL) or from T-cell precursors (T-ALL). Normally, B cells mature in the bone marrow and T cells mature in the thymus, so BCP-ALL originates in the bone marrow while T-ALL originates in the thymus. Leukemic cells retain the natural ability of lymphocytes to migrate in the body through the circulatory and lymphatic systems, and from their place of origin they expand to other organs. The main symptoms of ALL result from the intense proliferation of leukemic cells in the bone marrow, impairing its function, which is the production of all blood cells. There is a severe shortage of red and white blood cells and platelets, with life-threatening consequences such as anemia, impaired immunity and blood clotting disorders. Leukemia cells also multiply in other organs (e.g. lymph nodes, spleen, central nervous system) and damage them. ALL progresses rapidly and, if left untreated, is fatal within weeks. Therefore, it is extremely important to quickly diagnose ALL and start the therapy, as well as to monitor the effectiveness of the treatment in order to classify the patient to the appropriate risk group and to adjust the treatment intensity. Monitoring the effectiveness of treatment is also very important in preventing the recurrence of leukemia.
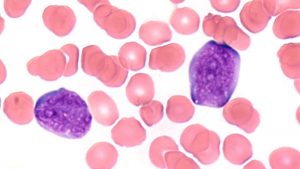 ALL might be described as an undesirable side effect of the very dynamic processes that take place in our immune system. B and T lymphocytes are capable of producing millions of different antibodies and receptors to defend us against the most diverse pathogens we may encounter. The number of genes in the human genome encoding antibodies and lymphocyte receptors is insufficient to produce such a wide variety. Therefore, it is necessary that genes encoding antibodies and lymphocyte receptors undergo rearrangements (Ig/TCR gene rearrangements). A natural element of these rearrangements is the occurrence of double-stranded DNA breaks, which are necessary, but also potentially dangerous. The aberrations which might accompany these DNA breaks can lead to the development of leukemia.
ALL might be described as an undesirable side effect of the very dynamic processes that take place in our immune system. B and T lymphocytes are capable of producing millions of different antibodies and receptors to defend us against the most diverse pathogens we may encounter. The number of genes in the human genome encoding antibodies and lymphocyte receptors is insufficient to produce such a wide variety. Therefore, it is necessary that genes encoding antibodies and lymphocyte receptors undergo rearrangements (Ig/TCR gene rearrangements). A natural element of these rearrangements is the occurrence of double-stranded DNA breaks, which are necessary, but also potentially dangerous. The aberrations which might accompany these DNA breaks can lead to the development of leukemia.
Ig/TCR rearrangements: genetic fingerprint of leukemia
However, we can use Ig/TCR gene rearrangements for diagnostic purposes. These rearrangements are unique and specific to the leukemia cells of a given patient, they serve as a “genetic fingerprint” of leukemia that allows the very sensitive detection of even a small number of leukemic cells. This is extremely useful for monitoring the effectiveness of ALL treatment, as even a small number of leukemia cells that have survived despite intensive treatment can initiate leukemia recurrence. This population of persistent leukemic cells is called Minimal Residual Disease (MRD). The MRD level is the most important prognostic factor in the treatment of ALL and it determines the intensity of treatment.
We were the first center in Poland to perform MRD tests in children treated for ALL. We determined the frequency of Ig/TCR gene rearrangements, which serve as markers for MRD monitoring, in Polish patients. We have developed and published guidelines for MRD testing, which facilitated the implementation in Poland of MRD diagnostic methods and standards, developed by the European consortium- the BIOMED Concerted Action. The activities of the Institute significantly contributed to the implementation of MRD tests in routine diagnostics in oncohematology.